Are Adaptogens the New Aspirin? A Look at the WHO’s Landmark Review of Herbal Supplements in 2025
I’ve long believed that herbal supplements live in a strange space — somewhere between tradition and speculation, between nature and marketing. And in 2025, that space just got a little less murky. Why? Because for the first time, the World Health Organization (WHO) has released a Global Review and Guidance Framework on Adaptogens — and it might be the most important development in the supplement space since omega-3s hit pharmacies.
As someone who has followed the supplement world for decades — and used many of them myself — I want to unpack what this means, why it matters, and what’s at stake. Because it’s not just a science story. It’s a story about trust, regulation, identity, and how we define "wellness" across borders.
From Folk Wisdom to Global Standards: Why the WHO’s Review Matters
If you’re new to the term “adaptogens,” let me briefly recap. Adaptogens are natural substances — typically plant-based — that are believed to help the body adapt to stress, fatigue, and general imbalance. Think ashwagandha, ginseng, rhodiola, holy basil. You’ve likely seen them in everything from teas to capsules to high-end beauty serums.
But while they’ve been used for centuries in Ayurvedic, Traditional Chinese Medicine (TCM), and Siberian herbalism, they’ve also existed in a legal and scientific gray zone. Are they food? Are they medicine? Are they… both?
That’s why the WHO’s 2025 review is so groundbreaking. It pulls together clinical data from 42 countries, compiles traditional use cases, and offers the first real framework to:
- Evaluate standardized dosages
- Recognize known interactions with medications
- Recommend safe upper limits
- Offer categorical efficacy ratings (mood, immune function, hormonal regulation, etc.)
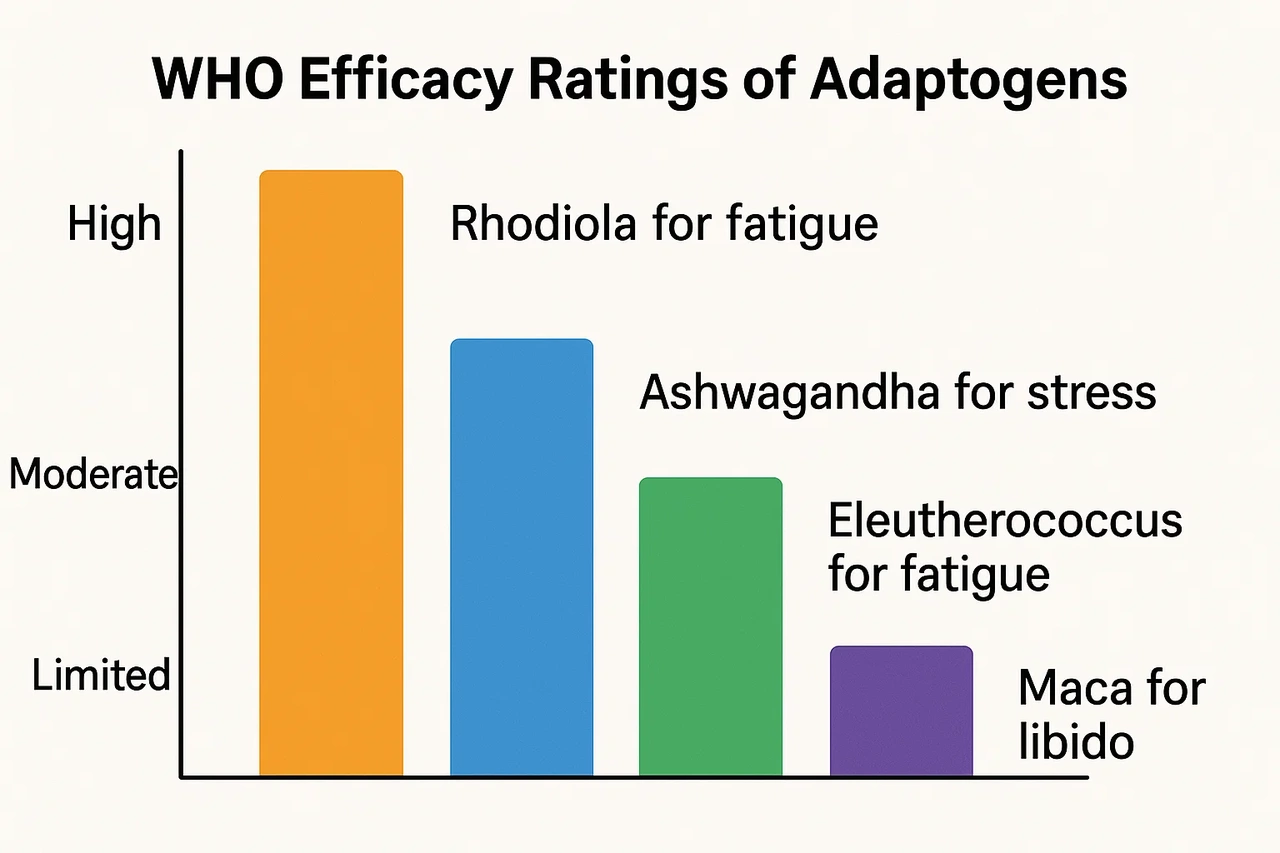
In essence, it’s asking the world to treat adaptogens with the seriousness they deserve — not as fringe add-ons, but as integrated health agents.
A Wake-Up Call for Supplement Brands
Here’s where things get interesting — and challenging. The WHO’s guidance isn’t legally binding (yet), but already governments in the EU, Australia, and parts of Asia are using it to redefine supplement regulations. Several brands are now facing new requirements around:
- Full botanical sourcing transparency
- Consistent phytochemical concentrations
- Third-party testing for heavy metals and pesticides
This isn’t just a paperwork exercise. It’s a real fork in the road for companies who’ve grown used to making vague claims under the umbrella of “traditional use.” I’ve spoken with several supplement founders in recent months, and there’s a mix of anxiety and optimism. The best ones — like Verdura Labs and BotaniCore — welcome the rigor. They’ve been trying to operate responsibly for years, and now they finally have a benchmark to distinguish themselves from the snake oil crowd. But others? Not so much.
Consumers Like Us: What This Means for Real People
I’ll be honest: I’ve used adaptogens for years. When I was going through perimenopause, ashwagandha genuinely helped me stay emotionally even. When I was recovering from burnout post-COVID, rhodiola helped me reclaim my mornings. But I’ve also had supplements that did nothing, or worse, triggered anxiety or heart palpitations. So this move toward clearer labeling, clinical backing, and global consistency feels… personal.

It’s a relief to think that soon, when I pick up a bottle of tulsi or schisandra, I might actually know:
- Where it came from
- What dose has been studied
- Whether it interacts with my thyroid meds.
That’s not a luxury. That’s basic health literacy. And we deserve it.
Culture, Power, and the Identity of Wellness
There’s another layer here I don’t want to ignore — cultural ownership. As adaptogens enter the Western scientific canon, there’s growing tension around who profits and whose knowledge is recognized. Are indigenous communities credited for their herbal wisdom? Are profits shared? Or are we seeing yet another case of global North extraction dressed up as progress? To their credit, the WHO has begun partnerships with community health leaders in India, Nepal, China, and Mexico to ensure that local voices remain central. But much more needs to be done. Ethical sourcing isn’t just about avoiding pesticides — it’s about honoring the ecosystems of knowledge that gave us these tools in the first place.
Looking Ahead: The Supplement Future I Want
I believe we’re on the edge of something hopeful. A world where supplements — especially herbal ones — are treated with both respect and rigor. Where science and tradition aren’t at war but in dialogue. Where a woman like me, navigating midlife with curiosity and caution, can make decisions rooted in evidence without losing wonder.
Here’s what I hope happens next:
- The U.S. follows suit and adopts WHO-informed labeling.
- Supplement makers invest in real studies, not influencer hype.
- More public education campaigns demystify adaptogens.
- Consumers — especially women — trust their intuition and ask better questions.
Because supplements aren’t magic bullets. But they can be part of a thoughtful, empowering, deeply personal health journey.

Close